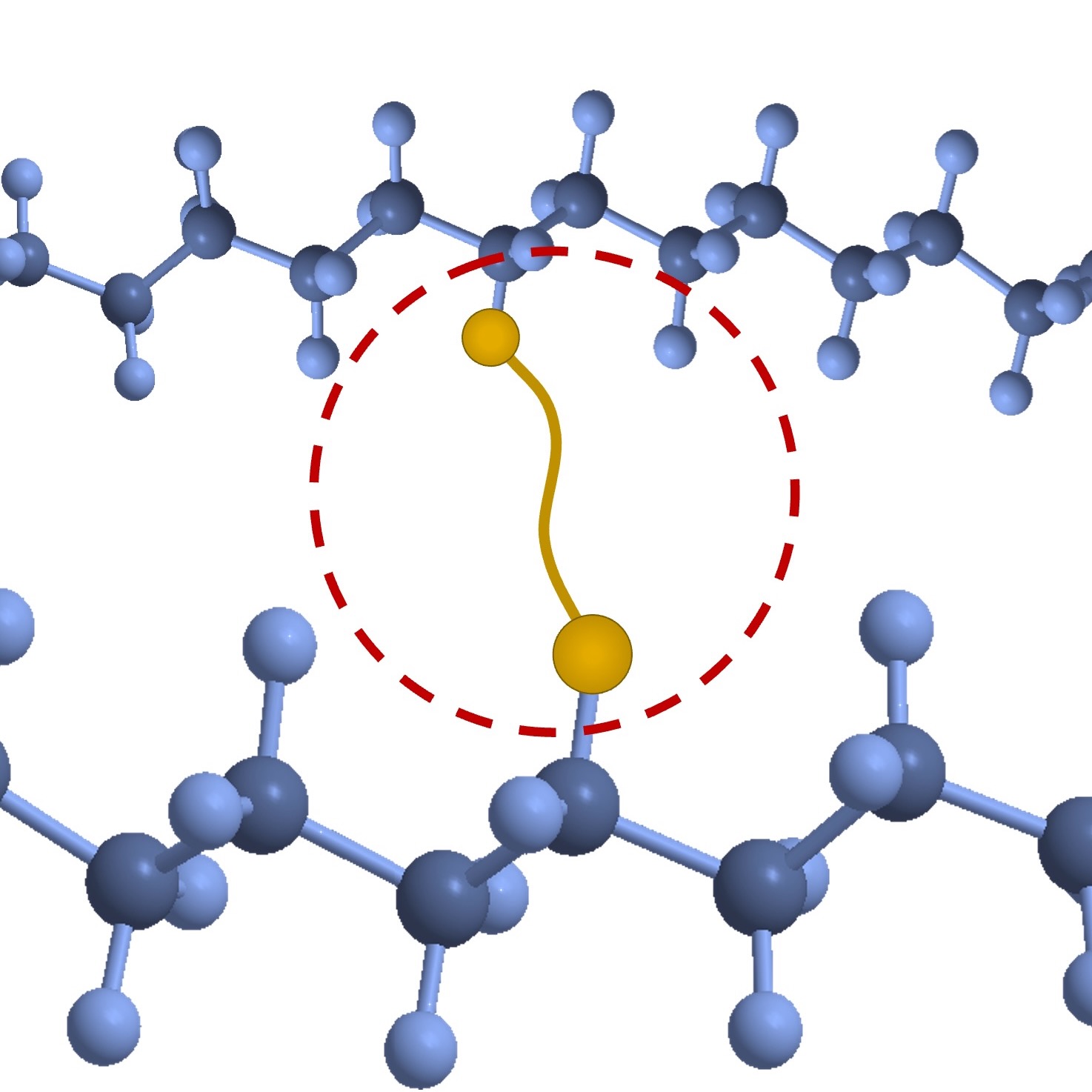
Crosslinking Overview

Drawing on inspiration from the field of chemical biology, XLYNX researchers overcame this bonding challenge by successfully applying a diazirine crosslinking solution to a materials science context. Diazirines are three-member rings with one carbon and two double-bonded nitrogen atoms. When exposed to moderate heat, UV light or near UV-light, diazirines rapidly break down, expelling a molecule of nitrogen gas to form a highly reactive carbene which is capable of forming C-H, O-H, or N-H insertion bonds with nearly any nearby polymer substrate. With high-intensity UV, this activation process can occur in seconds.
The science at the core of XLYNX’s products have been proven and peer-reviewed at the highest levels, and the adhesive and material improvements require only topical application and curing to achieve. Discover how crosslinking technology can resolve your design and manufacturing challenges.